DANVERS, Mass.--(BUSINESS WIRE)--Nov. 14, 2019-- To mark the five year anniversary of the study by Stretch, et al., on cost and outcomes trends for short-term mechanical circulatory support, Abiomed announces a comprehensive publication review of cost and comparative effectiveness of Impella in high-risk PCI and cardiogenic shock. The data, from a robust body of US and European evidence from 2004 - 2019, includes the PROTECT II FDA randomized controlled trial, data from the Centers for Medicare & Medicaid Services MedPAR database and more than 20 peer-reviewed clinical publications on cost-effectiveness. It demonstrates that Impella use in high-risk PCI (Protected PCI) and cardiogenic shock, when compared to intra-aortic balloon pump (IABP) or other therapies, is associated with improved patient outcomes and reduced costs. The review will be presented at upcoming meetings of interventional cardiologists and cardiac surgeons in Munich, Germany, and Phoenix, Arizona.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20191114005513/en/
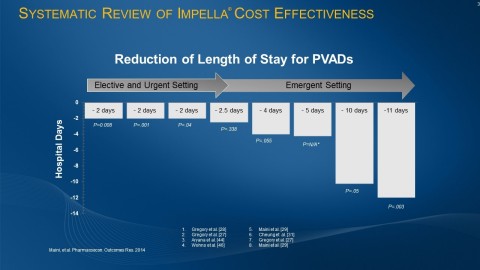
Multiple studies, including systematic reviews of multiple cost-effectiveness publications, demonstrate that Impella use is associated with a reduction in length of stay for patients, with a greater opportunity for benefit as the illness level increases.
Key evidence of Impella's cost-effectiveness includes data on how:
- Impella reduces mortality and cost
- Impella reduces length of stay and readmissions
- Impella reduces long-term health care costs
Impella Reduces Mortality and Cost
Several studies show that the use of percutaneous ventricular assist devices (PVADs), including Impella, is particularly cost-effective in cases of cardiogenic shock:
- A study conducted by Yale University colleagues Stretch, et al., found that in cases of cardiogenic shock in coronary atherosclerosis and other heart disease, PVADs reduced costs by $45,000 and $54,000 per case, respectively, and reduced mortality by 58% (Stretch, et al., Journal of the American College of Cardiology, 2014)
- For patients in cardiogenic shock requiring emergent hemodynamic support, PVAD therapy (and Impella 2.5 in particular) resulted in better outcomes, shorter length of stay, lower costs and a survival benefit when compared with surgical hemodynamic support alternatives (Maini, et al., Journal of Catheterization and Cardiovascular Interventions, 2014)
- Research by Vetrovec, et al, found that use of PVADs, including Impella, is associated with reduced mortality rates, shorter length of stay and lower hospital costs compared to ECMO. Their data showed that PVAD use compared to ECMO resulted in total episode-of-care (EOC) savings of $54,571 (Vetrovec, et al., Journal of Catheterization and Cardiovascular Interventions, 2019)
Impella Reduces Length of Stay and Readmissions
Several studies, including systematic reviews of multiple cost-effectiveness publications, demonstrate that Impella use is associated with a reduction in length of stay for patients, with a greater opportunity for benefit as the illness level increases1.
According to peer-reviewed published data, Impella use is associated with:
- A 52% reduction in repeated admission for revascularization at 90 days, as demonstrated in an independent economic analysis of the PROTECT II Randomized Controlled Trial (Gregory, et al., American Health & Drug Benefits Journal, 2013)
- A reduction in hospital stays ranging from 2-12 days (Maini, et al., Expert Review of Pharmacoeconomics & Outcomes Research, 2014)
- Shorter ablation times and reduced hospital length of stay in patients with unstable ventricular tachycardia (Aryana, et al., Heart Rhythm Society, 2014)
- A reduction in acute kidney injury, with average cost savings of $22,023 per case (Silver, et al., Journal of Hospital Medicine, 2016)
These findings are consistent in the United States and Europe. A retrospective cost-effectiveness analysis based on the USPella and Europella databases concluded that Impella is a cost-effective intervention compared with IABP for high-risk PCI patients (Roos, et al., Journal of Medical Economics, 2013).
As an example of Impella’s cost-effectiveness, the United Kingdom’s National Institute for Health Care and Excellence (NICE), one of the world’s most conservative regulatory bodies, confirmed the use of Impella in certain high-risk PCI patients.
“Sometimes trying to save costs by avoiding or delaying the use of innovative technologies sounds good, but you delay safe and effective therapy. Then the patients are sicker, and their outcomes are worse, which ends up being more costly for the patient and healthcare system. Using a better therapy up front can give you a better long-term outcome while reducing total costs,” said George Vetrovec, MD, professor, emeritus, at Virginia Commonwealth University.
Case Studies: Impella Reduces Long-term Health Care Costs
Because Impella enables the heart to rest and recover - while helping to restore native heart function - it may prevent the need for an implantable left ventricular assist device (LVAD) or a heart transplant, leading to an estimated $887,000 reduction in hospital charges over the period from 30 days pre-transplant to 180 days post-transplant discharge2. This makes native heart recovery one of the most cost-effective therapies in healthcare.
The case of Adam Millar illustrates the real-world benefit of native heart recovery. In 2018, Millar was 18 years old when Nima Aghili, MD, an interventional cardiologist at St. Anthony’s Central in Lakewood, Colorado, placed an Impella CP and Impella RP to recover Adam’s heart after he was diagnosed with atrial fibrillation and developed cardiogenic shock. At the time, Millar was a junior hockey player, and native heart recovery proved to be the best outcome for his health and future quality of life. Adam’s treatment was covered by United Healthcare.
“Before Impella recovered my heart, physicians considered me as a candidate for an implantable LVAD and a heart transplant. Both would have drastically decreased my quality of life,” said Millar. “If I received a heart transplant, I would have had a long recovery and taken auto-immune medication to avoid the rejection of the transplanted heart for the rest of my life.”
Impella also enables high-risk PCI patients to improve their native heart function through Impella-supported Protected PCI. The story of Jim Hoag illustrates how Protected PCI with Impella and complete revascularization enable better outcomes and improved quality of life for patients.
Jim, a 67-year-old grandfather, struggled with such extreme weakness and fatigue that he could barely walk from the parking lot to the athletic field to watch his grandsons play sports. He went to Spectrum Health in Grand Rapids, Michigan, where a diagnostic catheterization revealed severe blockages and poor heart function with an ejection fraction of 25%. Jim was referred to the advanced heart failure clinic where he was identified by the heart team as an appropriate candidate for Protected PCI. Drs. David Wohns and Kevin Wolschleger implanted the Impella 2.5 to support Jim's weak heart while they placed multiple stents.
Jim was discharged home one day later, and within two months his heart function had returned to near normal with an ejection fraction of 55%. Jim’s treatment was reimbursed by Medicare. Today, Jim feels better than he has in years, attended Abiomed's patient summit in Danvers, Massachusetts, this summer, and now climbs to the top of the stands to cheer on his grandchildren.
Cost- Effectiveness Compared to Other Therapies
Impella’s cost-effectiveness when compared to other related treatments, including LVADs, is validated based on an incremental cost-effectiveness ratio -- or ICER. ICER is a standard economic metric that represents the additional cost of one unit of a healthcare outcome, such as a quality-adjusted life year, gained by a healthcare intervention or strategy when compared with the next best alternative or standard of care. Amounts less than $100,000 are considered cost effective in the U.S., and less than $50,000 in most other countries. Impella’s ICER shows a reduction in costs of $135,000 per year in an emergent population3.
1 Nalluri, et al, Expert Review of Medical Devices, 2017; Aryana, et al, Heart Rhythm Society, 2014; Krenn, et al, SCAI, 2014; Maini, et al, Journal of Catheterization and Cardiovascular Interventions, 2014; Maini, et al, Expert Review of Pharmacoeconomics & Outcomes Research, 2014; Wohns et al, International Society for Minimally Invasive Cardiothoracic Surgery, 2014; Gregory, et al, American Health & Drug Benefits Journal, 2013; Gregory, et al, Journal of Managed Care Medicine, 2013; O’Neil,l et al, Journal of American College of Cardiology. USPella, 2013; Roos, et al, Journal of Medical Economics, 2013; Lamarche, et al, Journal of Thoracic and Cardiovascular Surgery, 2010
2Milliman 2017 US Organ and Tissue Transplant Cost Estimates and Discussion
3 Maini et al, Journal of Catheterization and Cardiovascular Interventions, 2014
ABOUT IMPELLA HEART PUMPS
The Impella 2.5® and Impella CP® devices are U.S. FDA PMA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI) such as stenting or balloon angioplasty, to re-open blocked coronary arteries. The Impella 2.5, Impella CP, Impella CP with SmartAssist®, Impella 5.0®, Impella LD®, and Impella 5.5™ with Smart Assist® are U.S. FDA approved heart pumps used to treat heart attack or cardiomyopathy patients in cardiogenic shock, and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart. The Impella RP® is U.S. FDA approved to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery. Impella is the most studied mechanical circulatory support device in the history of the FDA with real world clinical data on more than 100,000 patients and more than 550 peer-reviewed publications.
In Europe, the Impella 2.5, Impella CP and Impella CP with SmartAssist are CE marked for treatment of high-risk PCI and AMI cardiogenic shock patients for up to 5 days. Impella 5.0 and Impella LD are CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 10 days. The Impella 5.5™ with Smart Assist® is CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 30 days. The Impella RP is CE marked to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, open-heart surgery, or refractory ventricular arrhythmia.
To learn more about the Impella platform of heart pumps, including their approved indications and important safety and risk information associated with the use of the devices, please visit www.impella.com.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed, Inc. is a leading provider of medical devices that provide circulatory support. Our products are designed to enable the heart to rest by improving blood flow and/or performing the pumping of the heart. For additional information, please visit: www.abiomed.com.
Abiomed, Impella, Impella 2.5, Impella 5.0, Impella LD, Impella CP, Impella RP, and Impella Connect are registered trademarks of Abiomed, Inc., and are registered in the U.S. and certain foreign countries. Impella BTR, Impella 5.5, Impella ECP, CVAD Study, and SmartAssist are pending trademarks of Abiomed, Inc.
FORWARD-LOOKING STATEMENTS
This release contains forward-looking statements, including statements regarding development of Abiomed's existing and new products, the company's progress toward commercial growth, and future opportunities and expected regulatory approvals. The company's actual results may differ materially from those anticipated in these forward-looking statements based upon a number of factors, including uncertainties associated with development, testing and related regulatory approvals, including the potential for future losses, complex manufacturing, high quality requirements, dependence on limited sources of supply, competition, technological change, government regulation, litigation matters, future capital needs and uncertainty of additional financing, and other risks and challenges detailed in the company's filings with the Securities and Exchange Commission, including the most recently filed Annual Report on Form 10-K and Quarterly Report on Form 10-Q. Readers are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this release. The company undertakes no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect events or circumstances that occur after the date of this release or to reflect the occurrence of unanticipated events.
