Data demonstrates survival benefit with the Impella RP Recover Right protocol
NEW ORLEANS, La.--(BUSINESS WIRE)--Mar. 18, 2019-- Abiomed (NASDAQ:ABMD), a leading provider of breakthrough heart support technologies and the maker of the Impella RP heart pump, announces that survival data from the 18 month post-approval study of 42 Impella RP patients was presented at the American College of Cardiology’s (ACC) 68th Annual Scientific Session in New Orleans. The Impella RP is the only percutaneous technology with FDA PMA approval for right heart support designated safe and effective. The table below summarizes the new post-approval study data and compares it to the FDA study results submitted for Impella RP’s PMA approval.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20190318005252/en/
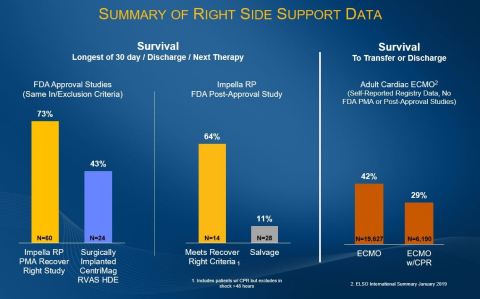
The Impella RP FDA post-market study data shows 64% survival rate for patients who meet the Recover Right criteria. (Photo: Abiomed, Inc.)
| |
|
|
|
|
|
|
|
|
|
|
Impella RP Post-Approval Study (PAS) Results with and without Recover Right Protocol
|
|
Post-Approval Study (PAS) – (N=42)
|
|
|
|
|
|
|
|
|
Survival Rates
|
|
Recover Right Protocol Population (N=14)
|
|
|
|
|
|
|
|
|
64% (9/14)
|
|
Salvage Patient Population (N=28)
|
|
|
|
|
|
|
|
|
11% (3/28)
|
| |
|
|
|
|
|
|
|
|
American College of Cardiology Scientific Session, 2019
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Impella RP Pre-market Approval (PMA) Study Results with Recover Right Protocol
|
| |
|
|
|
|
|
|
|
|
Survival Rates
|
|
PMA Subjects (RR + CAP + HDE PAS) – full cohort (N=60)
|
|
|
|
|
|
|
|
|
73% (44/60)
|
|
Cohort A – LVAD Patients (N=31)
|
|
|
|
|
|
|
|
|
77% (24/31)
|
|
Cohort B – Shock Patients (N=29)
|
|
|
|
|
|
|
|
|
69% (20/29)
|
| |
|
|
|
|
|
|
|
|
Journal of Heart and Lung Transplant, December 2018 (37)
|
| |
|
|
|
|
|
|
|
|
|
The post-approval study data was reviewed and submitted to the FDA by Abiomed on Wednesday, March 13, 2019. The FDA has confirmed the classification of patients into two categories: Recover Right protocol and salvage support. The Recover Right protocol includes patients who met the inclusion and exclusion criteria of the Recover Right FDA PMA clinical trial for Impella RP. The FDA also recognizes salvage patients as those outside the Recover Right protocol (>48 hours in cardiogenic shock from right side failure.)
The baseline characteristics of the two populations were different with higher mortality in the salvage group. Salvage patients are some of the sickest patients in the hospital and many have suffered out-of-hospital cardiac arrest or may have been transferred to multiple hospitals before receiving Impella RP. The FDA and Abiomed believe that physicians should have the ability to attempt lifesaving recovery measures on these patients based on their best judgement. Tim Deits, a 16-year-old Impella RP patient from Huntington Beach, Calif., is an example of an extremely sick patient who experienced an unwitnessed out-of-hospital cardiac arrest event, received CPR, and survived with recovery of his native heart.
The Impella RP post-approval study data compares to a survival rate of 73% in the Impella RP PMA Study, 42% for surgically implanted CentriMag RVAS HDE study protocol, and 29% - 42% self-reported survival to transfer or discharge for adult cardiac ECMO patients1. To note, the Recover Right protocol matches the CentriMag surgical protocol defined by the FDA as right-side failure. The Impella RP is the only device submitting post-approval study data on real-world patient outcomes, including salvage utilization. This patient population will continue to be studied in the ongoing cVAD Study.
“When a patient is in right heart failure, Impella RP allows the heart to rest and recovers the heart’s ability to pump blood,” said David Wohns, MD, Chief of Cardiology at Spectrum Health. “The Impella RP is an effective treatment for patients who receive a timely implant and meet the Recover Right inclusion and exclusion criteria.”
“The post-approval study data is analogous to our own independent data from multiple hospitals in the Cardiogenic Shock Working Group, which found an approximate 80% survival rate when the Impella RP was used in cardiogenic shock patients who met inclusion criteria from the Recover Right Study,” said Navin Kapur, MD, Executive Director of the CardioVascular Center for Research and Innovation at Tufts Medical Center. “Data like these highlight how the use of algorithms to recognize right sided failure and protocols for early hemodynamic support can help improve outcomes for cardiogenic shock patients.”
Abiomed encourages clinicians to review proper inclusion and exclusion criteria for Impella RP and to follow guidelines, which recommend implantation of Impella RP within 48 hours of cardiogenic shock onset caused by right side failure. Abiomed also encourages the use of best practices such as the National Cardiogenic Shock Initiative and the Shock Care Pathway Algorithms. Abiomed is committed to improving outcomes by leading in data collection within our commercial IQ Database and cVAD post-approval studies in order to identify and validate best practices.
ABOUT IMPELLA HEART PUMPS
The Impella 2.5 and Impella CP devices are FDA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI) such as stenting or balloon angioplasty, to re-open blocked coronary arteries. The Impella 2.5®, Impella CP®, Impella CP® with SmartAssist, Impella 5.0® and Impella LD® are FDA approved heart pumps used to treat heart attack or cardiomyopathy patients in cardiogenic shock, and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart. The Impella RP® is FDA approved to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery. To learn more about the Impella platform of heart pumps, including their approved indications and important safety and risk information associated with the use of the devices, please visit: www.protectedpci.com.
The Abiomed logo, Abiomed, Impella, Impella 2.5, Impella 5.0, Impella LD, Impella CP, Impella RP, Impella Connect, and Recovering hearts. Saving lives. are registered trademarks of Abiomed, Inc. in the United States and in certain foreign countries.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed, Inc. is a leading provider of medical devices that provide circulatory support. Our products are designed to enable the heart to rest by improving blood flow and/or performing the pumping of the heart. For additional information, please visit: www.abiomed.com.
FORWARD-LOOKING STATEMENTS
This release contains forward-looking statements, including statements regarding development of Abiomed's existing and new products, the company's progress toward commercial growth, and future opportunities and expected regulatory approvals. The company's actual results may differ materially from those anticipated in these forward-looking statements based upon a number of factors, including uncertainties associated with development, testing and related regulatory approvals, including the potential for future losses, complex manufacturing, high quality requirements, dependence on limited sources of supply, competition, technological change, government regulation, litigation matters, future capital needs and uncertainty of additional financing, and other risks and challenges detailed in the company's filings with the Securities and Exchange Commission, including the most recently filed Annual Report on Form 10-K and Quarterly Report on Form 10-Q. Readers are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this release. The company undertakes no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect events or circumstances that occur after the date of this release or to reflect the occurrence of unanticipated events.
1 2019 ELSO International Summary
